diabetes rfid chip The U.S. Food and Drug Administration today approved the Eversense Continuous Glucose . $10.99
0 · FDA approves first continuous glucose monitoring system with a
1 · FDA Approves Eversense 6
An optimal option for both advanced users and beginners. NFC & RFID allows you to read and write NFC tags. Work with QR codes. You can also read them or create new ones. Use a personal server to store scans of .
FDA approves first continuous glucose monitoring system with a
The U.S. Food and Drug Administration today approved the Eversense Continuous Glucose .The U.S. Food and Drug Administration today approved the Eversense Continuous Glucose Monitoring (CGM) system for use in people 18 years of age and older with diabetes. After a long wait, on Feb. 11, 2022, the company announced Food and Drug Administration (FDA) approval of its Eversense E3 version that can stay in the body for a full 6 months — rather than.
Sure, the technology—a millimeters-long microchip equipped with near-field communication capabilities and lodged just under the skin—had a niche, cutting-edge appeal, but in practical terms, a.
The Seattle-based company unveiled the prototype for its non-invasive, portable glucose monitoring device. The device incorporates Know Labs’ proprietary body-radio frequency identification (Bio.
The Bluetooth chip is connected to pins PA2 and PA3 of the microcontroller to achieve wireless transmission to a cell phone.Stelo is a wearable glucose biosensor designed for people living with type 2 diabetes not on insulin, and the first OTC CGM available without prescription. Learn more. Know Labs. Seattle, Washington-based Know Labs is developing two devices that employ Body-Radio Frequency Identification (Bio-RFID) technology, which uses radio waves to measure specific.
This work presents an integrated system-on-chip (SoC) that forms the core of a long-term, fully implantable, battery assisted, passive continuous glucose monitor (Fig. 1). It uses a 13.56 MHz, Industrial Scientific and Medical band (ISM) RF signal to harvest energy and communicate data to an interrogating reader.
Learn about our diabetic products including FreeStyle Libre – flash glucose monitoring, FreeStyle Navigator II – continuous glucose monitoring and FreeStyle Optium / Precision Neo - blood glucose and ketone monitoring system. The RFID microchip quickly and accurately transmits the glucose data back to a wireless scanner that displays the glucose level. The RFID microchip is powered by the scanner signal, avoiding the need for a battery in the microchip.
The U.S. Food and Drug Administration today approved the Eversense Continuous Glucose Monitoring (CGM) system for use in people 18 years of age and older with diabetes. After a long wait, on Feb. 11, 2022, the company announced Food and Drug Administration (FDA) approval of its Eversense E3 version that can stay in the body for a full 6 months — rather than. Sure, the technology—a millimeters-long microchip equipped with near-field communication capabilities and lodged just under the skin—had a niche, cutting-edge appeal, but in practical terms, a. The Seattle-based company unveiled the prototype for its non-invasive, portable glucose monitoring device. The device incorporates Know Labs’ proprietary body-radio frequency identification (Bio.
The Bluetooth chip is connected to pins PA2 and PA3 of the microcontroller to achieve wireless transmission to a cell phone.Stelo is a wearable glucose biosensor designed for people living with type 2 diabetes not on insulin, and the first OTC CGM available without prescription. Learn more.
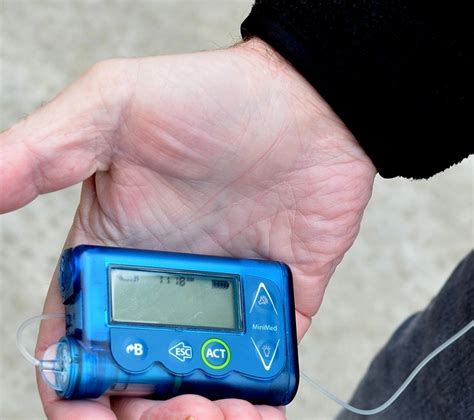
FDA Approves Eversense 6
Know Labs. Seattle, Washington-based Know Labs is developing two devices that employ Body-Radio Frequency Identification (Bio-RFID) technology, which uses radio waves to measure specific.This work presents an integrated system-on-chip (SoC) that forms the core of a long-term, fully implantable, battery assisted, passive continuous glucose monitor (Fig. 1). It uses a 13.56 MHz, Industrial Scientific and Medical band (ISM) RF signal to harvest energy and communicate data to an interrogating reader.Learn about our diabetic products including FreeStyle Libre – flash glucose monitoring, FreeStyle Navigator II – continuous glucose monitoring and FreeStyle Optium / Precision Neo - blood glucose and ketone monitoring system.

AFAIK the phones use a hardware called NFC controller in order to simulatate contactless .
diabetes rfid chip|FDA approves first continuous glucose monitoring system with a